
Competence development and assessment in the 6 rights of safe medicines management.
safeGUARD will provide learners with a comprehensive understanding of the 6 rights of safe medicines management and their importance in maintaining patient safety throughout the medicines management processes.
- Right documentation
- Right patient
- Right drug
- Right dose
- Right route of administration
- Right time of administration

SAFEGUARD MEDICINES MANAGEMENT CONTINUUM MODEL
The safeGUARD Medicines Management Continuum Model considers three key phases of medicines management and the role of key members of the multi-disciplinary healthcare team in maintaining patient safety at each phase.
- History Taking and Prescribing Phase
- Pharmacy Dispensing Phase
- Medication Administration Phase
DEVELOP AND ASSESS COMPETENCE USING A CLINICAL CASE STUDY APPROACH
safeGUARD adopts a clinical case study approach to undertake a stepwise review of important information in the clinical setting at each phase of the medicines management process. Learners will consider the role of 3 key members of the multi-disciplinary healthcare team and understand how error might be introduced at each phase. safeGUARD will provide a logical, comprehensive and effective framework for the identification of such errors and for taking appropriate actions to maintain patient safety. Each case study will require learners to assess, verify and implement the patient-safety critical requirements of the medicines management process in order to demonstrate their competence.

HISTORY TAKING AND PRESCRIBING PHASE
The role of the prescribing officer.
Understand the role of the prescribing officer in assessing the patient, reaching a decision on clinical treatment, accurately calculating and prescribing an appropriate medication and confirming the accuracy of information documented on the medication order.

PHARMACY DISPENSING PHASE
The role of the dispensing pharmacist.
Understand and consider the role of the dispensing pharmacist in checking and confirming the accuracy of the information documented on the medication order, advising on the safety and administration of the medication and performing any additional checks that must be made prior to dispensing the prescribed medication.
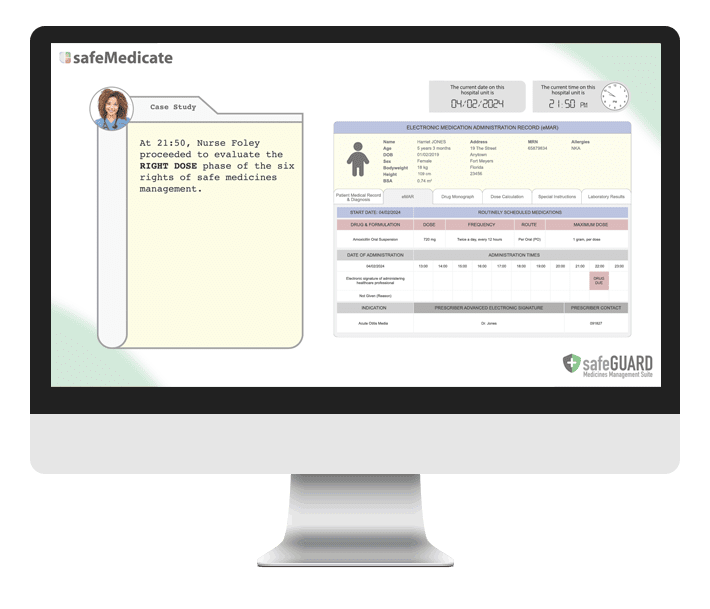
MEDICATION ADMINISTRATION PHASE
The role of the administering healthcare professional.
Understand and consider the role of the healthcare professional in checking and validating the prescribed and dispensed medication, calculating, preparing and measuring the dose to administer and complying with the 6 rights of safe medicines management throughout the medication administration phase.

ORGANISATION OF THE SAFEGUARD EDUCATION, LEARNING SUPPORT & ASSESSMENT ENVIRONMENT
safeGUARD enables learners to develop and demonstrate competence in safe medicines management by following a systematic journey that exposes them to each of its key components.
Education and Learning Support Environment
- Medicines Management Continuum Model
- Swiss Cheese Model of Medication Error Causation, Detection and Prevention Framework
- safeGUARD Medicines Management Competence Development Model: The 6 Rights
- safeGUARD Exemplar Patient-Based Case Studies in Medicines Management
Additional key resources include:
- Healthcare Measurement Units & Abbreviations
- Time & Frequency of Medication Administration & Abbreviations
- Routes of Medication Administration & Abbreviations
- Decimal Number and Dose Designations
- safeMedicate Rounding Rules Guidelines
Diagnostic Assessment Environment
safeGUARD presents each problem as an unfolding medicines management clinical case study. Each case study requires the learner to assess, verify and implement the patient-safety critical requirements of the medicines management process. Learners will be assessed via:
- Adult Based Patient Case Studies
- Child Bodyweight Based Patient Case Studies
The safeGUARD Medicines Management Suite builds upon and complements the drug dosage and prescribing calculation competence development and assessment featured elsewhere in safeMedicate and presents the most complete and comprehensive understanding and assessment of the 6 rights of safe medicines management available.
